Lessons from the COVID-19 service, part 1
Is this COVID patient’s AKI caused by the SARS-CoV-2 virus?
There are numerous people on our team with elevations in blood urea nitrogen and creatinine, suggesting a high prevalence of acute kidney injury (AKI) in COVID patients. Of course, it is well known that generally speaking, the population of people sick enough to be hospitalized from pretty much any cause is at higher risk of AKI anyway1.
While discussing an elderly patient with COVID who has concurrent AKI, the question arose today of whether COVID can cause AKI through mechanisms other than classical prerenal azotemia. Though there continues to be extremely limited data, a recent study from China reveals some insights into interactions between SARS-CoV-2 and the kidney2. A post-mortem analysis of 26 critically ill patients demonstrated heterogeneous findings of kidney pathology.
Light microscopy showed proximal acute tubule injury and sometimes frank necrosis. A couple samples had erythrocyte aggregation and obstruction, possibly related to concomitant bacterial infection. Other samples showed evidence of rhabdomyolysis. ACE II staining showed prominent ACE II expression in proximal tubular cells, much higher compared to that found in an archival biopsy of a nondiabetic, nonhypertensive patient without COVID. Electron microscopy found viral particles in the renal proximal tubular epithelium and podocytes (below; red points to virus, green points to spikes on the virus).
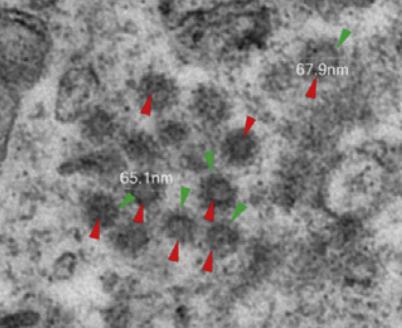
These findings suggest that SARS-CoV-2 can directly infect cells in the kidney. But it’s hard to say with this small uncontrolled study whether this is relevant to the clinical presentation, since so many other things can cause AKI (medication, prerenal azotemia, a background of CKD, obstruction). This study also only looked at patients who ultimately died, so their disease severity only represents the worst cases and are not indicative of COVID in the general population. Finally, I do not know whether infectivity of renal cells is a common trait of other coronaviruses (or even of other classes of virus), though the upregulation of ACE II may be interesting.
In the end, the clinical context of a patient’s AKI drives its management. In this particular patient, there are numerous reasons to suspect prerenal azotemia, so aggressive fluid management will outweigh all other modes of management unless there is a compelling reason for another underlying mechanism of kidney injury.